Reports of research work funded by grants prior to 2012
Malaghan Institute of Medical Research
Fluorescent Probes to study Glycolipid uptake and trafficking in Cancer Immunotherapy
BL Stocker, MSM Timmer
Immunoglycomics Research Group
In this research project, we investigated the development of different fluorescent dyes to study glycolipid uptake and trafficking in Cancer Immunotherapy. This work builds on our previous synthesis of the a-galactosyl ceramide derivative containing the ‘dansyl’ fluorophore 1 (Figure 1). Although dansyl-a-GalCer had use for the in vitro assessment of glycolipid uptake by antigen presenting cells (APCs), the use of this fluorescent derivative in vivo was limited due to the low quantum yield (F) of the dansyl dye. Accordingly, a-galactosyl ceramide derivatives containing alternative (brighter) flurophores at the 6´-position were required.
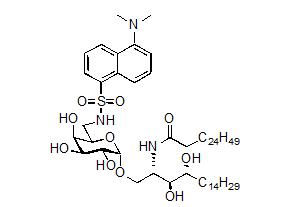
Figure 1. Dansyl-a-GalCer
To this end, we prepared a variety of new fluorescent groups based on the ‘Bodipy’ fluorophore 2 (A, Figure 2). These ‘bodipy’ groups contain a ‘linker’ motif, which allows their ready attachment to glycolipids (such as a-GalCer), and include lipophilic derivatives (e.g. 2a) and those containing water-soluble sulfonate groups (e.g. 2b and 2c). While some further optimisation of the yield for the final sulfonation process is required, our overall synthetic routes is short and the dyes that have been prepared can be readily coupled to 6-azido-a-GalCer to prepare fluorescent-bodipy-a-GalCer derivatives. Moreover, the quantum yield for representative bodipy derivatives is good (F = 0.35 – 0.57), with compounds being highly coloured and fluorescent (as illustrated B, Figure 2).
 Figure 2. A. Representative ‘bodipy’ dyes. B. Fluorescent microscopy images of BODIPY succinimidyl ester for conjugation to 6-azido-a-GalCer
Figure 2. A. Representative ‘bodipy’ dyes. B. Fluorescent microscopy images of BODIPY succinimidyl ester for conjugation to 6-azido-a-GalCer



