Reports of research work funded by grants prior to 2013
Malaghan Institute of Medical Research
The impact of obesity and hyperuricemia on inflammatory immune regulation
J Harper
Background
Obesity and hyperuricemia are key risk factors in the development of gouty arthritis. The initiation of the immune response to the inflammatory trigger, monosodium urate (MSU) crystals, is highly dependent on the pro-inflammatory activation of resident macrophages. Clinical studies indicate that adipose resident macrophages in obese patients are skewed towards an inflammatory resting phenotype compared to lean controls. Currently, there is little information available on how obesity and hyperuricaemia affects resident macrophage populations outside the adipose tissue.
The aim of this study was to investigate the impact of central obesity and hyperuricaemia on MSU crystal-induced inflammation in a murine model of gout.
Results
Due to difficulties in obtaining the PoundTM mice and unforseen breeding problems, PoundTM mice have only recently become available for experimental work. Profiling of the inflammatory phenotype of these mice is now underway. To offset this setback we established an alternative diet-induced obesity model to obtain data on the inflammatory phenotype of non-adipose resident macrophages.
High fat diet induced weight gain and insulin resistance
Normal C57BL6/J mice that were fed either a high fat diet (HFD, 23.5% fat (HFD) or control diet (NFD, 5.3% fat) over 12 weeks. HFD mice gained significantly more weight than NFD mice that was associated with increased visceral fat, elevated serum insulin levels and a reduced ability to regulate blood glucose. These data established that the HFD mice developed a pre-diabetic phenotype of metabolic syndrome.
Effect of HFD-induced obesity on naive resident macrophage inflammatory phenotype
Purification of naïve resident macrophages followed by MSU crystal challenge ex vivo showed that IL-6, MCP-1, KC and GM-CSF release by unstimulated resident macrophages from obese mice were significantly elevated but dropped upon MSU crystal stimulation ex vivo (Figures 1A and data not shown). In contrast IL-1β production by naïve mice was unchanged but was significantly elevated for MSU-stimulated resident macrophages from obese mice compared to NFD controls (Figure 1B).
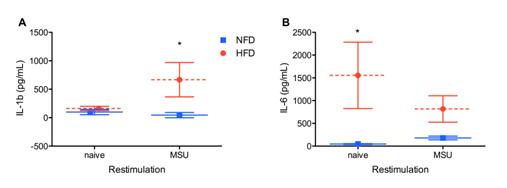
Figure 1: High fat diet drives a more pro-inflammatory resting macrophage phenotype.
Effect of HFD-induced obesity on MSU crystal-induced inflammatory in vivo
We observed elevated levels of IL-6, KC and MCP-1 in the peritoneal wash supernatants from naïve, obese mice compared to controls (Figures 1A-D, 0 hours). After MSU challenge in vivo, there was a similar increase in IL-1β levels for both diet groups but the elevated cytokines observed in naïve obese mice decreased over time. Interestingly, there was a trend towards decreased numbers of resident macrophages and MSU-crystal recruited monocytes and neutrophils in obese mice compared to controls (Figures 2E,F).
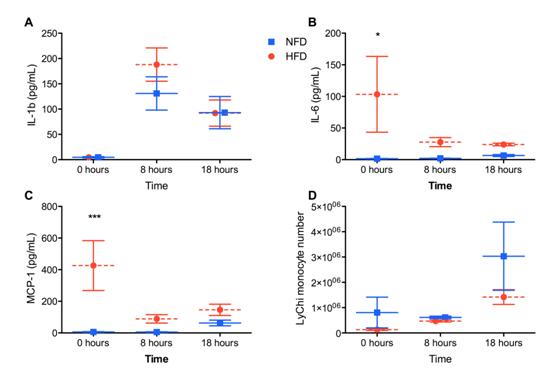
Figure 2: High fat diet does not alter inflammatory cell recruitment or cytokine production following MSU challenge in vivo.
Conclusions
In summary, our findings show that diet-induced obesity can raise the inflammatory phenotype of unstimulated resident macrophages outside the adipose tissue. This inflammatory phenotype may be linked with high levels of local GM-CSF, which is linked with the development of M1-like proinflammatory macrophages. However, although these resident macrophages exhibit an exacerbated IL-1β response to MSU crystal stimulation ex vivo, this does not translate into overall elevated inflammatory responses following in vivo MSU crystal stimulation.
This work has been submitted for presentation at the 2013 American College of Rheumatology Conference. A manuscript, “Diet-induced obesity induces pro-inflammatory resident macrophages”, has also been prepared for submission to the journal Annals of Rheumatic Diseases.



