Reports of research work funded by grants prior to 2016
Victoria University of Wellington
Toll-like receptor 9 expression and activation in acute coronary syndrome patients on dual anti-platelet therapy
K Hally
Clinical Research Laboratory
Introduction
Thrombotic response to atherosclerotic plaque rupture or erosion is the major underlying cause of acute coronary syndromes (ACS). Platelet activation and aggregation plays a pivotal role in the formation of arterial thrombi in ACS. Dual antiplatelet therapy (DAPT), a combination of aspirin with a P2Y12 receptor antagonist, has been shown to substantially reduce the risk of adverse cardiovascular events and is the current standard of care for ACS. However, a proportion of patients will continue to experience recurrent ischaemic events despite treatment with DAPT. Because platelet activation can occur via multiple signalling pathways, alternative activation pathways that are not currently therapeutically targeted may contribute to this ongoing ischaemic risk in some patients. Platelet toll-like receptors (TLRs) may represent such an alternative pathway.
TLRs are known to be expressed on and within platelets and various immune-driven, anti-microbial effects have been attributed to platelet-TLR agonism. Most papers have reported TLR expression and activation in small cohorts of healthy volunteers but little information is available on how these parameters are modulated in ACS.
The grant given by WMRF in June last year allowed us to pursue the following aims, adapted from the original application submitted in March:
- We aimed to examine baseline TLR9 expression in healthy subjects and in ACS subjects treated with DAPT and how TLR9 expression changes in response to platelet activation in both cohorts
- Following on from this, we aimed to examine platelet activation in response to ODN2006 agonism, a TLR9 agonist, in both cohorts.
- Separately, we aimed to examine baseline TLR 1, 2, 4 and 6 expression in ACS subjects in comparison to both healthy subjects and stable coronary artery disease (CAD) subjects.
TLR9 is the least well-characterised platelet-TLR but is known to be present in platelets TLR9-mediated platelet activation in response to free radical-altered self-ligands has recently been characterised. TLRs 1, 2 and 6 are expressed on the platelet surface and are known to be pro-atherogenic. TLR2 dimerises with either TLRs 1 or 6 to recognize different bacterial lipopeptides and TLR2/1 agonism induces platelet activation. TLR4 is the most well-characterised platelet-TLR. Significant platelet cell-surface TLR4 expression was reported and LPS administration induces platelet activation and aggregation.
Methods
- TLR9 expression was assessed in both resting and thrombin receptor activator peptide (TRAP)-activated platelets (1 and 10 µM) from 5 healthy and 5 ACS subjects treated with DAPT. TLR9 expression was measured using median fluorescent intensity (MFI) by flow cytometry. TRAP is a universal platelet agonist; 1 and 10 µM TRAP results in low and maximal platelet activation, respectively.
- In a separate cohort of 16 healthy subjects and 16 ACS subjects treated with DAPT, ODN2006-mediated platelet activation (5 µM) was examined in whole blood (WB) and platelet-rich plasma (PRP) using cell-surface CD62p and CD63 expression by flow cytometry. CD63 and CD62p positivity indicates an activated platelet. CD63 was measured using MFI and CD62p was measured using the percentage of the platelet population that was CD62p-positive.
- We are currently optimising a western blotting protocol for assessing baseline TLR 1, 2, 4 and 6 expression. Whole platelet lysates have been collected from 20 ACS and 20 age- and gender-matched healthy and stable CAD subjects.
Results
- Baseline TLR9 expression in healthy subjects and in ACS subjects treated with DAPT. TLR9 expression in resting ACS platelets (MFI, 15.1±1.1) was significantly greater compared to healthy subjects (MFI 7.2±2.5, p<0.01; Figure 1). In the healthy cohort, a dose-dependent increase in TLR9 expression was reported for 1 µM TRAP-activated platelets (MFI 9.8±2.7, ns) and 10 µM TRAP-activated platelets (MFI 12.8±3.6, p<0.05) in comparison to unstimulated platelets. This trend was not observed in ACS subjects. TLR9 expression following 1 µM (MFI 14.27±2.50) or 10 µM (MFI 14.77±3.86) TRAP activation was similar to TLR9 expression seen in unstimulated platelets.
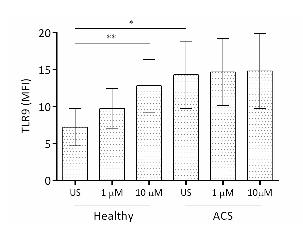
Fig 1. TLR9 expression increases in ACS platelets and upon platelet activation, compared to resting healthy platelets.
Platelet-TLR9 expression, as measured by MFI, was analysed in response to 1 µM and 10 µM TRAP stimulation. Mean ± SD for 5 subjects in each cohort are shown. One way ANOVA was found to be significant (p<0.05) for each parameter and allowed for Student’s t-tests to be performed. * p<0.05, ** p<0.01.
- Platelet activation following ODN2006 agonism.
- Healthy subjects. ODN2006 resulted in a significant increase in the percentage of CD62p-positive platelets (55% increase from no agonist sample, p<0.0001) in healthy WB. Similarly, a significant increase in CD63 expression was observed across the whole platelet population (MFI 0.61 in no agonist vs 3.81 in ODN2006 stimulated sample, p<0.0001). Similar to the response in WB, ODN2006 stimulation significantly increased CD62p (46% increase, p<0.0001) and CD63 expression (MFI 1.63 vs 3.17, p<0.001) in healthy PRP in comparison to unstimulated platelets.
- ACS subjects. In response to ODN2006, there was a significant increase in both CD62p (61% increase from no agonist sample, p<0.0001) and CD63 expression (MFI 0.75 in no agonist vs 4.84 in ODN2006 stimulated sample, p<0.0001) in ACS WB when compared to unstimulated platelets. Similarly, platelet activation in ACS PRP was significantly larger than that observed in the no agonist sample for both CD62p (47% increase, p<0.0001) and CD63 (MFI 0.67 vs 2.41, p<0.0001).
- Baseline TLR 1, 2, 4 and 6 expression in healthy vs. stable CAD vs. ACS subjects. The western blotting method is currently being optimized for detection of these TLRs. Atthistage, we are able to qualitatively detect an increase in all TLRs in ACS platelets when compared to age-matched healthy platelets (Figure 2). We are hoping to have this project completed by the end of August.
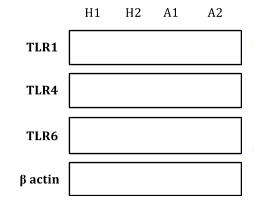
- TLR expression increases in baseline ACS platelets when compared to healthy platelets.
Western blot analysis for TLRs 1, 4, and 6 in unstimulated whole platelet lysates for healthy subjects (H1, H2) and ACS subjects (A1, A2).
Conclusion
We have demonstrated that TLR9 expression was significantly elevated in ACS, compared to healthy platelets. This may indicate increased sensitivity to TLR9 agonists and increased platelet reactivity in these patients. Platelet activation caused increased TLR9 expression in healthy platelets. This is a potential mechanism to explain increased platelet-TLR9 expression in ACS patients who undergo extensive platelet activation during their event. ODN2006-mediated platelet activation in PRP demonstrates the functionality of platelet-TLR9. We are the first to demonstrate ODN2006-mediated platelet activation in ACS patients on DAPT, indicating that the TLR9 pathway is inadequately inhibited by current anti-platelet drugs. Platelet-TLR9 appears to be a novel connection between oxidative stress, infection and platelet activation. Activation of this pathway potentially increases the risk of thrombosis and the risk of adverse cardiovascular events.



